Regulatory Science for Engineering Intuitive, Engaging, Safe and Effective Human-Device Interaction
(Contreras-Vidal, UH BMI Lab; Kimberly Kontson, FDA)
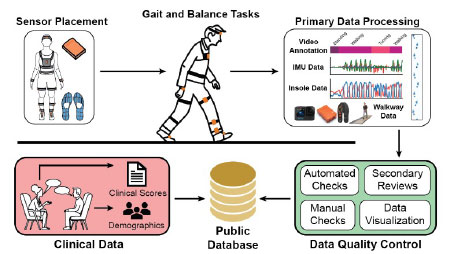
Background: Interest in wearables as stand-alone medical devices to monitor changes in gait impairment and/or to assess the efficacy of therapeutic interventions is on the rise. However, algorithms lack validation in large scale, independent studies. The main deliverable of this project is an open access database of synchronized raw inertial measurement unit (IMUs), insole pressure sensor, and pressure sensing walkway data, and clinical information from individuals with Parkinson’s Disease (PD) at various stages of the disease and age-matched controls. This will benefit FDA and other stakeholders by providing a tool to independently validate algorithms used to support clinical endpoints and the means to identify novel digital biomarkers for PD.
Research Plan: The role of the REU student(s) will be to characterize wearable sensors for gait assessment and to curate a database of wearables data from individuals with Parkinson’s Disease. Activities may include experimental design, experimental setup, data acquisition, writing MATLAB scripts for data analysis, and mastering the use of relevant lab instrumentation. The learning objectives of this research project are to (1) learn about Parkinsonian gait and emerging technologies used to assess gait disorders, (2) learn how to acquire and analyze data from various wearable sensor modalities, (3) develop new or further develop skills related to human subject research protocol development and implementation, and (4) develop communication, organizational, and other critical skills necessary for successful execution of a multi-site clinical study.
Prerequisites: An introductory course in biomechanics and knowledge of at least one scientific programming language are desirable.

Dr. Kimberly Kontson received her B.S. from the Pennsylvania State University, State College, PA and Ph.D. from the University of Maryland, College Park, MD in Biomedical Engineering. She is currently a biomedical engineer with the U.S. FDA’s Center for Devices and Radiological Health, Office of Science and Engineering Laboratories (OSEL), and also serves as a subject matter expert in human factors/usability engineering to the Office of Product Evaluation and Quality Human Factors Review Team. As the program coordinator for the OSEL Human-Device Interaction research program, Dr. Kontson's current research interests include development of regulatory science tools to support evaluation of wearable technologies, clinical outcome assessment development for advanced prosthetic devices, and evaluation of virtual reality devices for rehabilitation.