Regulatory Science for Engineering Intuitive, Engaging, Safe and Effective Human-Device Interaction
(Ji Chen, UH CEMC/BRAIN; Dr. Omar Al Kalaa, Electromagnetic Compatibility Lab)
Background: The fifth generation (5G) cellular communication technology introduces technical advances that can expand medical device access to connectivity services. However, assessing the safety and effectiveness of emerging 5G-enabled medical devices is challenging as relevant evaluation methods have not yet been established. FDA’s Office of Science and Engineering Labs (OSEL) has developed and published a design model for 5G testbed as a regulatory science tool (TRUST) for assessing 5G connectivity enablers of medical device functions. OSEL also integrated a TRUST deployment using commercial grade and emulated 5G equipment, allowing end-to-end over-the-air 5G connectivity.
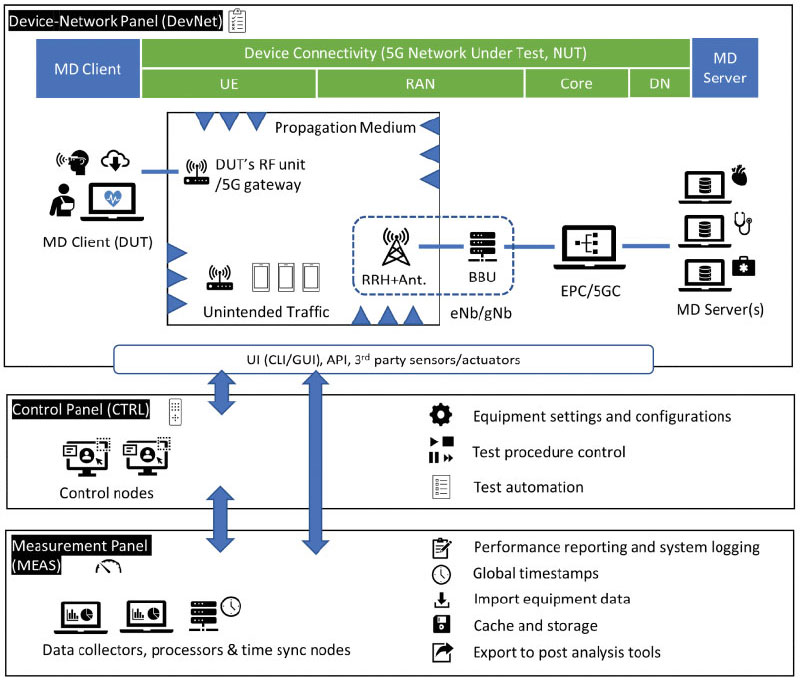
Research Plan: REU students will be mentored by OSEL staff members (all PhDs) to select, deploy, and test publicly available open-source software components that emulate parts of the 5G network. The students will integrate the open-source components with the existing lab 5G testbed and help identify and overcome interoperability issues.
Prerequisites: An introductory course in Linux is required. Desired course but not required: Python, C++, and communication networks.

Dr. Mohamad Omar Al Kalaa is an Electrical Engineer with the Center for Devices and Radiological Health (CDRH) at the U.S. Food and Drug Administration (FDA), where he leads research activities focused on medical device connectivity. He received his Ph.D. in electrical and computer engineering from the University of Oklahoma, Norman, OK, USA, in 2016. His research interests include healthcare applications enabled by wireless technology, wireless coexistence of technologies in unlicensed bands, and wireless medical device testing methodologies. Dr. Al Kalaa served as the co-chair of the Medical Device Innovation Consortium (MDIC) 5G-enabled medical device working group and as the secretary of the ANSI C63.27 standard for evaluation of wireless coexistence working group